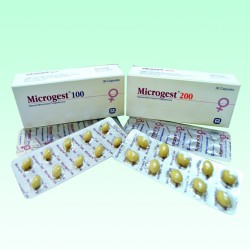
Microgest 200mg Soft Gel Capsule 10's Strip

Microgest 200mg Soft Gel Capsule 10's Strip
৳ 300.60/=
Ex Tax: ৳ 300.60/=
- Stock: In Stock
- Model: Medi-01022
- Generic: Progesterone
- Pack Size: Each pack contains Microgest 200mg Soft Gel Capsule 10's Strip
0 items sold
7680 views
Available Options
Indications
Progesterone capsules are indicated for use in the prevention of endometrial hyperplasia in nonhysterectomized postmenopausal women who are receiving conjugated estrogens tablets. They are also indicated for use in secondary amenorrhea.
Therapeutic Class
Drugs for menopausal symptoms: Hormone replacement therapy, Female Sex hormones, Oral Contraceptive preparations
Pharmacology
Progesterone is the main hormone secreted by corpus luteum. It induces secretory changes in the endometrium, promotes mammary gland development, relaxes uterus, blocks follicular maturation and ovulation, and maintains pregnancy.
Dosage & Administration
Oral administration:
Prevention Of Endometrial Hyperplasia: Progesterone Capsules should be given as a single daily dose at bedtime, 200 mg orally for 12 days sequentially per 28-day cycle, to a postmenopausal woman with a uterus who is receiving daily conjugated estrogens tablets.Treatment Of Secondary Amenorrhea: Progesterone Capsules may be given as a single daily dose of 400 mg at bedtime for 10 days. Some women may experience difficulty swallowing Progesterone Capsules. For these women, Progesterone Capsules should be taken with a glass of water while in the standing position.
Vaginal or rectal insertion:
For women undergoing Assisted Reproductive Technology (ART) programme: The recommended dose is 400 mg twice a day by vaginal insertion. Start using Cyclogest 400 mg on the day of egg retrieval. The administration of Cyclogest should be continued for 38 days if pregnancy has been confirmed.For the treatment of premenstrual syndrome and post-natal depression: The recommended dose is 200 mg once a day or 400 mg twice a day by vaginal or rectal insertion.
The pessary may be inserted into either the vagina or rectum (back passage) depending upon the following certain other conditions.
Interaction
Enhanced clearance with enzyme-inducing drugs eg, carbamazepine, griseofulvin, phenobarbital, phenytoin and rifampicin. Ketoconazole may increase serum levels of progesterone. May inhibit ciclosporin metabolism.
Contraindications
Progesterone Capsules should not be used in women with any of the following conditions:
- Progesterone Capsules should not be used in patients with known hypersensitivity to its ingredients. Progesterone Capsules contain peanut oil and should never be used by patients allergic to peanuts.
- Undiagnosed abnormal genital bleeding.
- Known, suspected, or history of breast cancer.
- Active deep vein thrombosis, pulmonary embolism or history of these conditions.
- Active arterial thromboembolic disease (for example, stroke and myocardial infarction), or a history of these conditions.
- Known liver dysfunction or disease.
- Known or suspected pregnancy.
Side Effects
Common side effects are Headache, Breast T enderness, Joint Pain, Depression, Dizziness, Urinary Problems, Abdominal Pain, Vaginal Discharge, Nausea / Vomiting, Worry, Chest Pain, Diarrhea, Night Sweats, Breast Pain, Swelling of Hands and Feet, Vaginal Dryness, Constipation, Breast Carcinoma, Breast Excisional Biopsy, Cholecystectomy
Pregnancy & Lactation
Pregnancy Category B. Reproductive studies have been performed in mice at doses up to 9 times the human oral dose, in rats at doses up to 44 times the human oral dose, in rabbits at a dose of 10 mcg/day delivered locally within the uterus by an implanted device, in guinea pigs at doses of approximately one-half the human oral dose and in rhesus monkeys at doses approximately the human dose, all based on body surface area, and have revealed little or no evidence of impaired fertility or harm to the fetus due to progesterone.
Nursing Women: Detectable amounts of progestin have been identified in the milk of nursing women receiving progestins. Caution should be exercised when Progesterone Capsules are administered to a nursing woman.
Nursing Women: Detectable amounts of progestin have been identified in the milk of nursing women receiving progestins. Caution should be exercised when Progesterone Capsules are administered to a nursing woman.
Precautions
Discontinue medications if there is sudden partial or complete loss of vision, proptosis or diplopia; migraine and embolic disorders; epilepsy, migraine, asthma, cardiac or renal dysfunction. History of depression, glucose tolerance and diabetic patients. May impair ability to drive or operate machinery. Avoid sudden withdrawal of progesterone; lactation.
Overdose Effects
No studies on overdosage have been conducted in humans. In the case of overdosage, Progesterone Capsules should be discontinued and the patient should be treated symptomatically.
Use in Special Population
Pediatric Use: Progesterone Capsules are not indicated in children. Clinical studies have not been conducted in the pediatric population.
Geriatric Use: There have not been sufficient numbers of geriatric women involved in clinical studies utilizing Progesterone Capsules to determine whether those over 65 years of age differ from younger subjects in their response to Progesterone Capsules.
Hepatic Insufficiency: The effect of hepatic impairment on the pharmacokinetics of Progesterone Capsules has not been studied.
Renal Insufficiency: The effect of renal impairment on the pharmacokinetics of Progesterone Capsules has not been studied.
Geriatric Use: There have not been sufficient numbers of geriatric women involved in clinical studies utilizing Progesterone Capsules to determine whether those over 65 years of age differ from younger subjects in their response to Progesterone Capsules.
Hepatic Insufficiency: The effect of hepatic impairment on the pharmacokinetics of Progesterone Capsules has not been studied.
Renal Insufficiency: The effect of renal impairment on the pharmacokinetics of Progesterone Capsules has not been studied.
Source: medex.com.bd